 |
 |
 |
 |
 |
 |
David dives in to see how we make water safe to drink.
Segment Length: 6:30
Drinking Water
Show Number 1413
How do we get the impurities
out of drinking water?
Where does drinking water come from?
![]() Getting Started
Getting Started
Water has been called the universal solvent because it can dissolve just about anything over time. How does the solubility of water change with temperature? See how much salt you can dissolve in equal volumes of water at different temperatures. How can you use your test results to make predictions about water solubility around the world?
Each day, millions of Americans use billions of gallons of water without knowing where it comes from or what might be in it. As populations grow, the combination of increased demand and increased pollution means many of us are using sources of water that are less than pristine. Contamination from sediment, bacteria, protozoans, heavy metals, and synthetic organic compounds shows up with alarming frequency. As a result, many municipalities are having to pre-treat drinking water.
The first step in most municipal treatment systems involves gravity. If you've ever let a glass of chocolate milk stand for any length of time, you've probably noticed that much of the chocolate settles to the bottom of the glass. The same is true of sediment in water. When the water is allowed to stand in large pools, many of these suspended particles simply settle to the bottom where they are collected and disposed of.
Next comes flocculation. Here, a chemical is added to the water that causes tiny suspended particles to clump together. Usually, these flocs settle out just like large-sized sediment. But if they escape, they are caught by filters farther down the line.
To kill unwanted microbes, protozoans, and other living organisms, many municipalities chlorinate the water. Chlorine is a chemical that kills microorganisms. In drinking water, the concentrations are low enough that often you can't even taste it.
The final stage in most municipal treatments is filtration and aeration. Water is pumped through large tanks filled with fine sand, called rapid sand filters. As the water flows through the spaces between the sand grains, suspended particles and dead microbes get trapped. Sometimes, crushed anthracite coal is used in addition to sand. Because the coal grains carry a charge on their surface, they act like tiny magnets attracting other charged contaminants. Aeration is exactly what it sounds like - adding air to water. When sprayed through large, fountainlike devices, water not only gains a great deal of oxygen, but any volatile compounds that may have been in the water escape into the atmosphere.
In the end, all the water we use doesn't just go down the drain and disappear. It gets recycled by the evaporative power of the sun. When water evaporates, most of the contaminants stay behind, which in theory means it rains pure water back on the land. Unfortunately, because of gases in the air, much of the water that falls from the sky is coming down pre-polluted, so municipalities are going to have to work even harder in the future to treat the water we drink.
Huggler, T.E. (1994, Apr) And not a drop to drink: Water treatment on a wilderness trip. Outdoor Life, p. 48.
Kotz, D. (1995, Nov) How safe is your drinking water?
Good Housekeeping, p. 128.Powledge, F. (1982) Water. New York: Farrar Straus Giroux Publishing.
Soda bottle hydrology. U.S. Department of Energy Publication DOE/EM-0215.
(301) 903-4000.Student activity sheets for drinking water projects. (1992, July)
U.S. Environmental Protection Agency Publication 810/F-92-003.
(202) 260-2090.3-2-1 Contact Extra: Down the drain. Available through WINGS for Learning,
(800) 321-7511.Hydrologic Information Unit
U.S. Geological Survey
419 National Center
Reston, VA 22092Soil and Water Conservation Society
7515 NE Ankeny Rd
Ankeny, IA 50021-9764
Student Activity:
Drinking Water
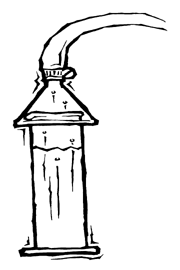
Still Waters
Calculate how much energy it takes to distill drinking water.
Since new sources of freshwater are hard to come by, some people have suggested using distilled seawater as a source of drinking water. In this activity, you will build a still and discover some of the costs associated with producing freshwater from the sea.
Materials
1. Pour 25 ml table salt into 1 liter of clean tap water in the larger beaker to make your simulated seawater. Pour the seawater into the flask.
2. Place one end of the plastic tubing over the narrow end of the funnel. It should fit snugly. If not, secure with a hose clamp or rubber bands.
3. Place the flask on the hot plate and invert the funnel so that the wide end sits over the top of the flask. Use a test-tube clamp on the ring stand to hold the funnel in place and run the other end of the tubing into the empty 500-ml beaker.
4. Record your start time and turn on the hot plate to its highest setting for 15 minutes. The water will begin to heat up and boil. After 15 minutes, turn off the hot plate. Allow the system to cool down for 10 minutes and then record how much water has collected in the beaker by pouring it into the graduated cylinder. Be careful! This water will still be hot. Use an insulated glove!
5. Check the bottom of the hot plate to see how many watts of electricity it uses. (If there is no power indication, use 1000 watts, which is average.) Calculate the total amount of energy used to distill your sample by multiplying the wattage by .25 hours (15 minutes). Convert this number to kilowatt hours by dividing it by 1000. Example: 1000 watts x .25/1000 = .25 kW hrs.
6. Based on your experiment, calculate how much electricity would be required to distill one full liter of seawater. To do this, take 1000 ml and divide it by the volume you collected, then multiply by the amount of power you used in kW hrs. Example: If you collected 100 ml of fresh water: 1000 ml/100 ml = 10 x .25 kW hrs = 2.5 kW hrs of electricity/liter. Then use your electric company's current rate per kilowatt hour to discover the cost.
Questions
![]()
Many people are serviced by municipal systems that get water from either
surface or underground sources. Many rural home owners have their own wells.
Discover the source of your drinking water and make a map of its path to
your tap.
![]()
Design your own solar still. Remember, the water cycle works because freshwater
is evaporated from the land and sea surface by the sun's heat. See if you
can design and build your own solar system to transform saltwater to fresh.
![]()
Why is the ocean salty if it is fed by rivers carrying freshwater? With
the exception of distilled water, all freshwater has some dissolved minerals
(salts) in it. To see how salty your water is, put one liter of water in
a clean saucepan and allow the entire volume to evaporate. The materials
left behind are the former minerals that were in solution.
Tapes of this episode of Newton's Apple and others are available
from GPN for only $24.95.
Please call 1-800-228-4630.
For information on other Newton's Apple resources for home and school,
please call 1-800-588-NEWTON!
 We encourage duplication for educational non-commercial use.
We encourage duplication for educational non-commercial use.
This activity has been copied, with permission, from the AskERIC server to ours, to allow faster access from our Web site. We encourage you to explore the Newton's Apple Teacher's Guide site.