
To Purchase NEWTON'S APPLE videos and other science stuff, call 1-800-588-NEWTON.
To begin the lesson, pose the following situation: On a bright, sunny day, you park your car in the sun and lock it. When you come back later, what has happened? Have you ever been inside a greenhouse when the sun was out? How did it feel? This phenomenon is called the greenhouse effect. Before showing the video, ask the following: Is Earth enclosed by something? In what ways is Earth like a greenhouse? What effects will changes in Earth's average temperature have? How do human activities contribute to this change in temperature? Are we making Earth too hot to live on? How can we cool off?
Climate that is, the weather over a long period depends on Earth's average temperature. This temperature stays relatively constant because Earth's surface absorbs energy from sunlight, changing it to heat (infrared radiation). Greenhouse gases, particularly water vapor, absorb the resulting heat energy and hold it in the atmosphere instead of allowing it to radiate out into space. (In an actual greenhouse, the glass windows block the heat's exit.) This greenhouse effect keeps us warm, but scientists are concerned that humans may be creating problems by adding certain greenhouse gases to the atmosphere, such as carbon dioxide (from carbon-based fuels), chlorofluorocarbons (from aerosol cans), and methane (from cow digestion). The accumulation of greenhouse gases could result in global warming an increase in the average temperature that would probably lead to climate change. If the worst predictions come true, we may have to deal with melting polar ice caps; rising sea levels; uninhabitable coastal areas (where half the world's population now lives); and wild, unpredictable storms. Agricultural areas might turn to desert, while barren areas might become fertile. Researchers have analyzed air that was trapped in glacial ice 160,000 years ago. By comparing that air to the air in our current atmosphere, they have discovered an increase in carbon dioxide as the use of fossil fuels has increased. Some scientists aren't convinced that excess greenhouse gases will actually cause global warming. They point out that cooling effects also are taking place. For instance, the oceans absorb much of the carbon dioxide that human activity contributes to the atmosphere. Higher temperatures cause more water to evaporate into clouds, which shade Earth from sunlight, cooling it. Particulate matter from volcanic eruptions and other pollutants deflects sunlight and also contributes to cooling. The greenhouse effect is a very complex issue. Much of the information we have about global warming comes from computer models that estimate climate change. These estimates may be inexact because the atmosphere is so huge. In addition, an observed temperature increase may be caused by something else. Some measurements suggest that variations in the sun's light output cause temperature changes far more significant than those caused by greenhouse gases.
1. Sometimes a waste product or pollutant can be recycled for another use. Can you think of some other uses for the greenhouse gases? 2. How can we determine if human activity is really contributing to global warming? And if it is, what should we do about it?
BUBBLES GREENHOUSE EFFECT: Student Activity Use vinegar to indentify carbonatecontaining materials.
Carbon dioxide doesn't just take up space in the atmosphere. It's part of the global "carbon cycle," in which carbon travels through the living environment to nonliving things and back again. You can also find carbon in some rocks and minerals, generally combined with oxygen and a metal in a compound called a carbonate. You can identify carbonate-containing materials because they react with acid to give off bubbles of carbon dioxide. The chemical sentence (equation) that describes this process is: Na2CO3 + 2HC2H3O2 > CO2 + H2O + 2NaC2H3O2 Materials
2. Put the material in the dish and place several drops of white vinegar on it. 3. Observe whether bubbles form. If bubbles do form, keep adding vinegar until they stop. 4. When bubbles stop forming, dry and weigh the material (if there is any left).
Questions 1. Which materials gave a positive test for carbonate? How can you be certain that any bubbles you saw were carbon dioxide and not some other gas? What percentage of the material was carbonate? 2. How else could you measure the amount of gas given off? 3. Does it seem likely that carbonatecontaining rock is a source of atmospheric carbon dioxide?
|
Books and articles Botkin, D. &
Keller, E. (1995) Rawls, R. (1996,
Nov 4) Roleff, T. (Ed.)
(1997) Sarmiento, J. &
Le Quere, C. (1996, Nov) Computer Software KIDWare: Web sites CIESIN Environmental Defense
Fund
Fill a 1-liter beaker with water. Add 7 drops of red food coloring. This makes a solution approximately 350 parts per million (the same as the carbon dioxide concentration in the atmosphere). Can you still see the red color? What if you double the concentration? Carbon dioxide is not a
pollutant in the traditional sense. All green plants need it to grow. Make
a list of other things that have this good-in-small-amounts/
NEWTON'S APPLE video cassettes and educational materials provide further information about this and other topics. Call 1-800-588-NEWTON.
|
||||||

|
|||||||




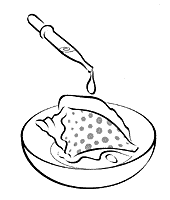 1. Weigh
the material to be tested on a balance.
1. Weigh
the material to be tested on a balance. 


