
Background A very approximate, but useful, description of white light such as the sunlight reaching the earth, is that the light is a mixture of light of several colors. Specifically, we can construct white light by overlapping three beams of light, one each of red, green, and blue. These are the additive primaries as shown in the figure below. Where all three overlap we see white light.

Those colors formed at the places where two light beams are overlapped are called the subtractive primaries. They are yellow (red + green), magenta (red + blue), and cyan (blue + green). Dyes of these colors are frequently used to make colored photographs and inks such as are found in Mr. Sketch pens. If a piece of yellow cellophane, for example, is placed in front of a white light source, such as a flashlight or slide projector, a beam of yellow light is formed. Since white light can be described as a mix of red, green and blue and since yellow is a mixture of red and green, but no blue, we can say that the yellow cellophane has allowed red and green light to pass through, but subtracted or absorbed the blue light.

Mixtures of subtractive primary dyes can be used to make inks of different colors. For example, it is common to prepare green ink by mixing yellow and cyan subtractive primary dyes. The origin of this color can be visualized by considering the effect of placing cyan and yellow cellophane filters in front of a white light source.

When dyes are mixed in the ink on a paper surface, light reflecting from the paper after passing through the dyes is now colored. In this example the cyan dye absorbs the red light and the yellow dye, the blue light. Only the green light is not absorbed and so the ink is seen as green.

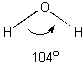
Paper Chromatography Paper chromatography is a method chemists use to separate compounds from one another, but not change them. In this section we shall explore how this separation is made--using different inks as mixtures and dyes as different compounds. Molecules with similar arrangements of their atoms or molecular structures are attracted to each other. Water molecules have the structure shown below in which the two hydrogen atoms form a 104o angle with the oxygen at the vertex.
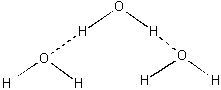
Because of this structure the oxygen end of the molecule has a small negative electrical charge and the hydrogen end has a small positive charge. Liquid water is held together by the attraction between the charges on different molecules. This is shown below for a small cluster of water molecules.
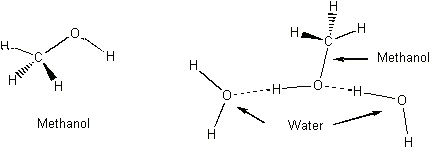
A molecule with these charged regions is called a polar molecule. Methanol (CH3OH) has a similar structure (see below), and the methanol molecules are very soluble in water because of the mutual attraction between the two polar molecules.
A more complex, yet still similar molecule is cellulose, a molecule which is the basic component of paper. It is a very long molecule (a polymer) in which thousands of rings of six atoms each are linked together like beads. A portion of a cellulose molecule is shown below.
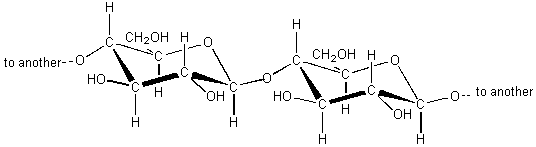
The polar -OH regions of these molecules are attracted to OH groups on adjacent cellulose chains helping to hold the fibers together in paper. Not surprisingly, water molecules, being polar, are also attracted to these regions and when paper is wet it loses strength because the water molecules get between the cellulose chains and weaken the attraction between them.
When the end of a piece of paper is dipped into water the water molecules keep finding new places (polar regions) to stick to and so the water molecules climb up the paper being replaced by new water molecules below. Other molecules which might be dissolved in the water will also be carried along up the paper. This is applied to the separation of dyes in a technique known as paper chromatography.
A spot of dye is placed on the paper above the level of the water. As the water moves up, the dye molecules will move with it if they are more strongly attracted to the water molecules than to the paper molecules. If the dye molecules are more strongly attracted to the paper than to the water, they will move more slowly than the water or even not at all. What if the dye is a mixture? If two or more dyes have been mixed to form an ink, then they may move at different rates as the water moves up the paper. If this happens, they will separate and we can identify them . This is depicted in the sketches below.

After running the chromatogram, each separated "spot" can be assigned a Retention Factor (RF) which is characteristic of the specific dye(s) associated with it. The RF is a ratio of the distance the "spot" travels relative to the distance the solvent (water in this case) travels. The RF is calculated by dividing the "spot" distance by the solvent distance. This ratio should be a constant that is characteristic of the dye(s) in a particular spot under a particular set of chromatographic conditions (i.e. paper chromatogram, water solvent, etc.)

We can use this as a tool to help answer several questions about the inks. These are listed in the Purpose section which follows.
Purpose
Procedure
Separate the dyes in the inks of the Mr. Sketch pens assigned to you
by your instructor using paper chromatography as outlined below.

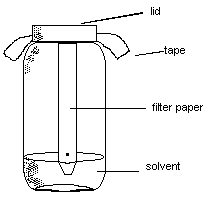
Repeat your experiment with the same pens you used with pure water.
Notes
This activity has been copied, with permission, from the California State University at Stanislaus server to ours, to allow faster access from our Web site. We encourage you to explore the original site