Purpose: This experiment is designed to illustrate the purpose of the different components of a photographic developer. It will also illustrate the chemistry of the toning process. Specifically, you will determine the role of one component in the developer and identify the importance of reaction duration as a variable in the toning of black and white prints. For the first part, be specific. Indicate which component you are omitting. Discuss the role of that component in your conclusion section.
Procedure: NOTE: Weigh out all of the materials needed for Section 2 before doing Section 1.
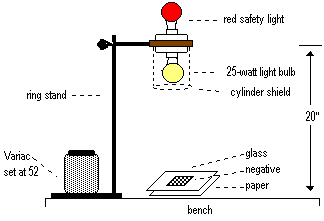
In the first section of this experiment you will determine a set of optimum conditions for developing a contact print using a light bulb for exposure and a standard, already-prepared developer. Two factors are important in determining the optimum conditions. The first is the amount of light hitting the photographic paper. This depends on the intensity of the light, the length of the exposure and the distance of the light from the paper. The greater the amount of light hitting the paper, the darker the print since more silver halide grains are exposed. This assumes all other conditions are held constant. The directions for the first part are listed in Section 1.
In the second section of the experiment you will prepare a developer
by weighing out and mixing the necessary chemicals. You will then use the
conditions determined in the first part to prepare a contact print with
your own developer. Most photographic developers have several components
in common. These are:
In your developer the chemicals will be:
Wastes: The developer and the stop bath can be put down the drain with the water running. Used fixer has silver in it and must be put in the acqueous waste container.
Section 1: Determining the Optimum Conditions
Steps:

Trial Distance to paper Variac setting Exposure time Developing time
1
2
3
4
5 (if necessary, or more)
Best conditions ____________________________________________________________
Section 2: Preparing your own developer
Steps:
What happens if...? What happens if you prepare a developer but leave out one of the ingredients? Try it and record your results.
Section 3: Toning [Note: these experiments can be performed in regular light].
Iron Toning: Prepare an iron toning bath by mixing 10.0 ml of ferric ammonium citrate (10% solution), 10.0 ml of K3Fe(CN)6 (10% solution) and 100 ml of a 10% solution of acetic acid in a 400-ml beaker. This solution can be safely disposed of in the sink.
Place a print in the iron toning solution for 5 minutes. What happens? How does the length of time in the toning solution affect the print? Test this by placing a print in the toning bath for only 2 minutes. Try another time for the toning bath. Rinse the print in deionized water briefly, and record your results.
Copper Toning: Prepare a copper toning bath as follows. Dissolve 0.54 g of K3Fe(CN)6 and 2.65 g of potassium citrate in 100 ml of H2O. In a separate beaker, dissolve 0.66 g of copper sulfate and 2.65 g of potassium citrate in 100 ml of water. Mix equal volumes of the two solutions just prior to use. (Copper is a heavy metal. Dispose of this solution in the aqueous waste container).
Place a print in your bath for 5 minutes and rinse What do you see? How does the length of time in the bath affect the result?
Sepia Toning: Place a print in a 400 ml beaker containing about 100 ml of 20% sodium thiosulfate solution for 5 minutes. Without rinsing, immerse the print in a beaker containing the prepared hydrochloric acid for 30 minutes then rinse in distilled water. (Both of these solutions can be put down the drain with the water running). What do you observe? How does length of time in the acid bath affect the result? (Note: In your Results section, include the prints by taping them in the appropriate section).
For your conclusion describe the role of the chemical which you left out of the developer. Refer to the mechanism of the action of the black and white developer as described by your lab instructor and in the handout. Also draw a conclusion about the effect of time on the progress of the toning reaction.
This activity has been copied, with permission, from the California State University at Stanislaus server to ours, to allow faster access from our Web site. We encourage you to explore the original site